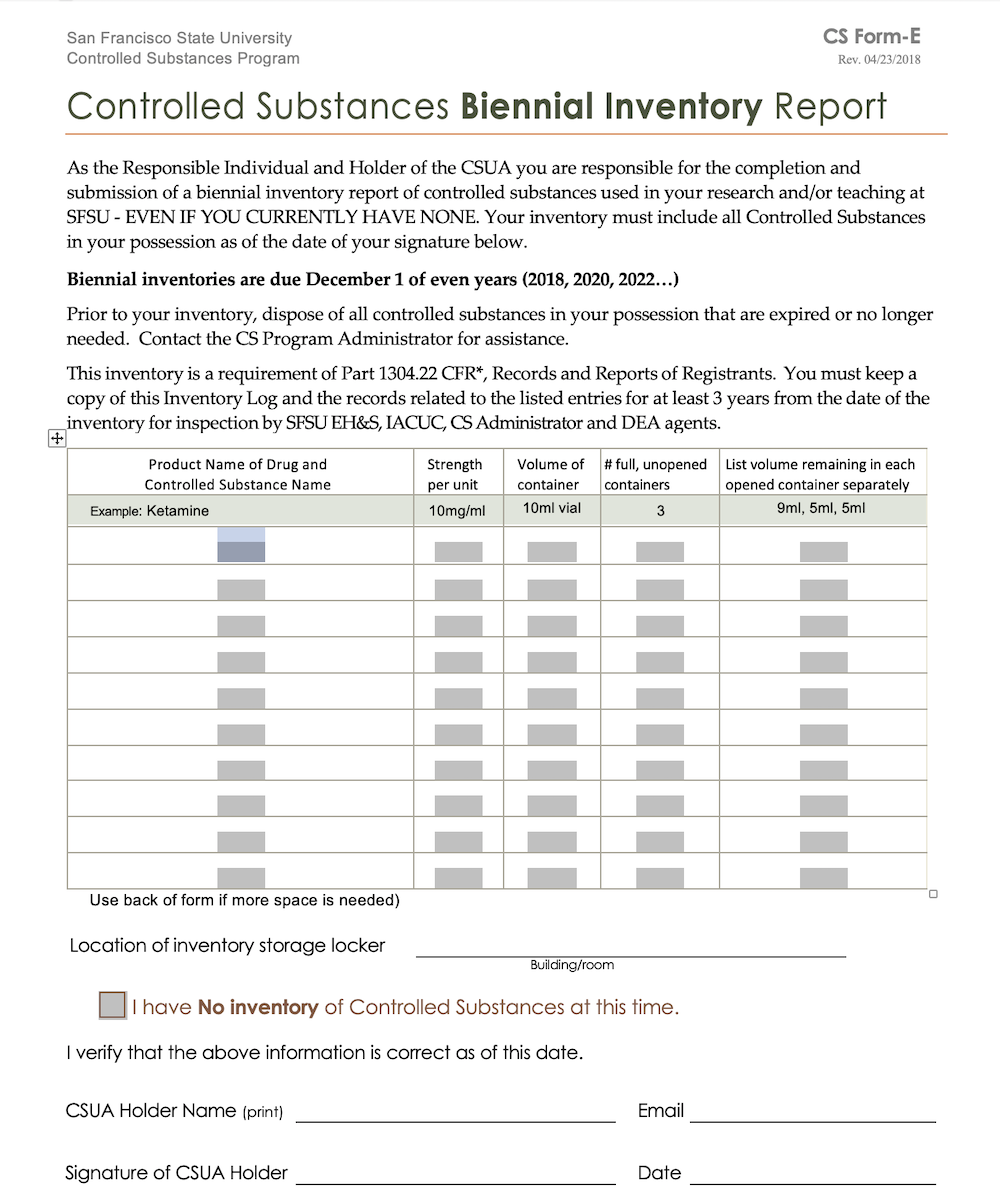
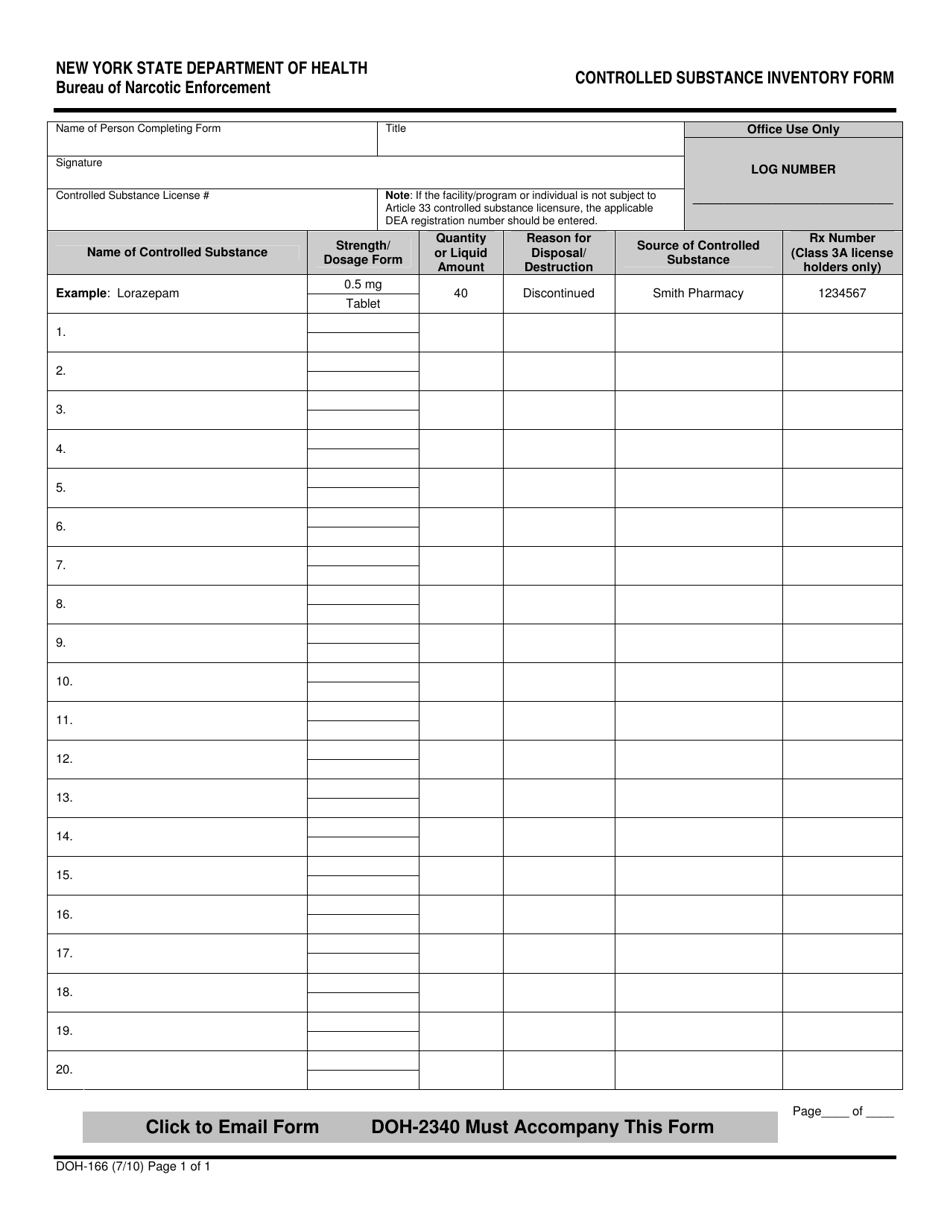
Dea Biennial Inventory Form
Dea Biennial Inventory Form - The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each.
Fillable Online Biennial & Initial Controlled Substance Inventory Form
(2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory.
Fillable Online DEA Controlled Substance Inventory Form Fax Email Print
(2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. The dea requires a physical.
Dea biennial inventory form Fill out & sign online DocHub
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and.
CS Form E Biennial Inventory Environment, Health and Safety
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. The dea requires a physical inventory of all controlled substances to be conducted.
Dea form 41 Fill out & sign online DocHub
(2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. (2) schedule i and ii drugs must be separated from.
Form DOH166 Fill Out, Sign Online and Download Fillable PDF, New
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical.
5 Best Printable Home Med Printablee 15048 Hot Sex Picture
The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must be separated from all other drugs or. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users.
Dea Controlled Substance Log Template JMT Printable Calendar
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. Dea biennial controlled substance inventory form for the use of controlled substances.
DEA Biennial Controlled Substance Inventory Form Fill and Sign
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); (2) schedule i and ii drugs must be separated from all other drugs or. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs.
SOLUTION Cs biennial inventory form Studypool
(2) schedule i and ii drugs must be separated from all other drugs or. The dea requires a physical inventory of all controlled substances to be conducted every two years for each registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Dea biennial controlled substance inventory form for the use of controlled substances.
(2) Schedule I And Ii Drugs Must Be Separated From All Other Drugs Or.
Dea biennial controlled substance inventory form for the use of controlled substances in research a separate initial inventory is required for each. Template (word) used to perform a biennial inventory of the controlled substances currently stored at the dea registered location. (2) schedule i and ii drugs must be separated from all other drugs or. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);