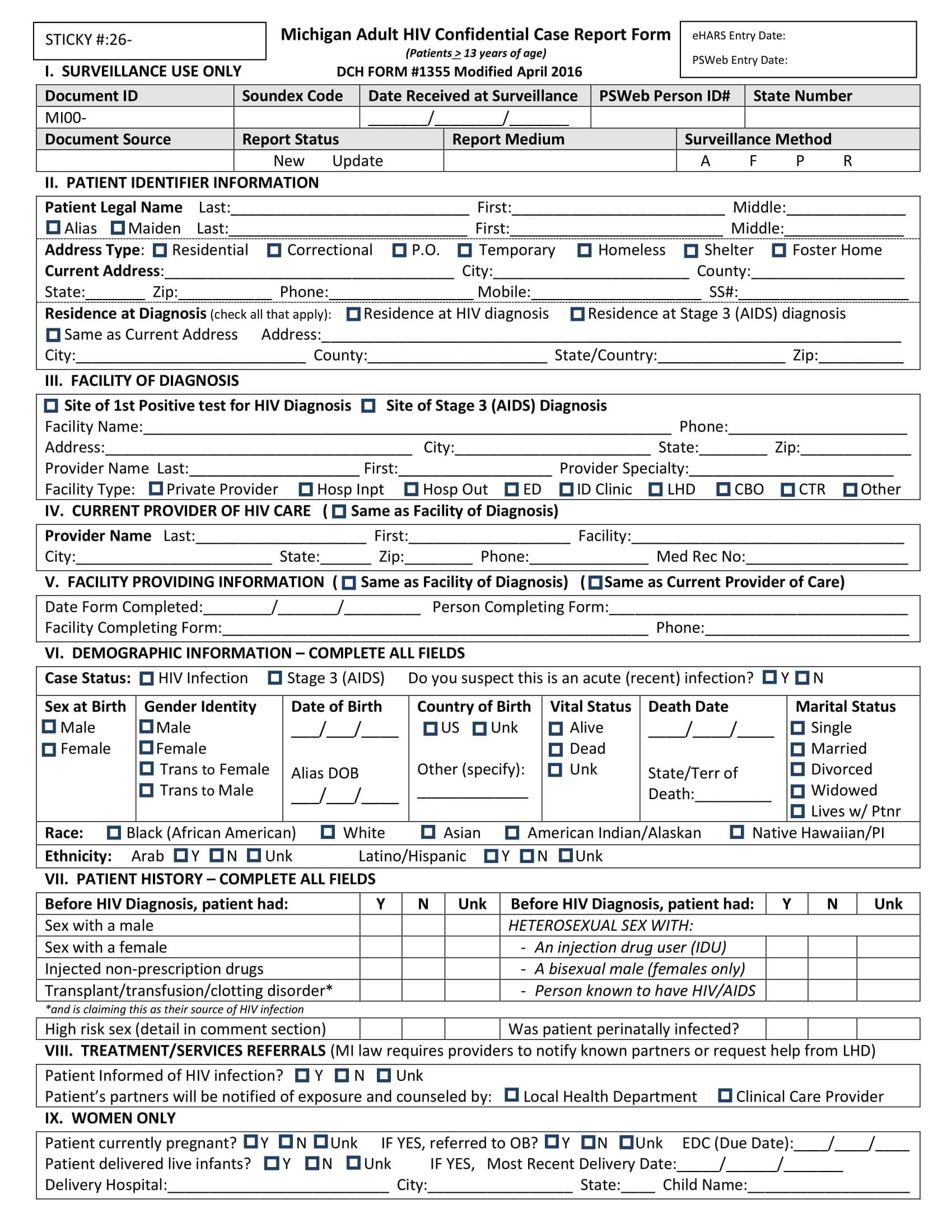
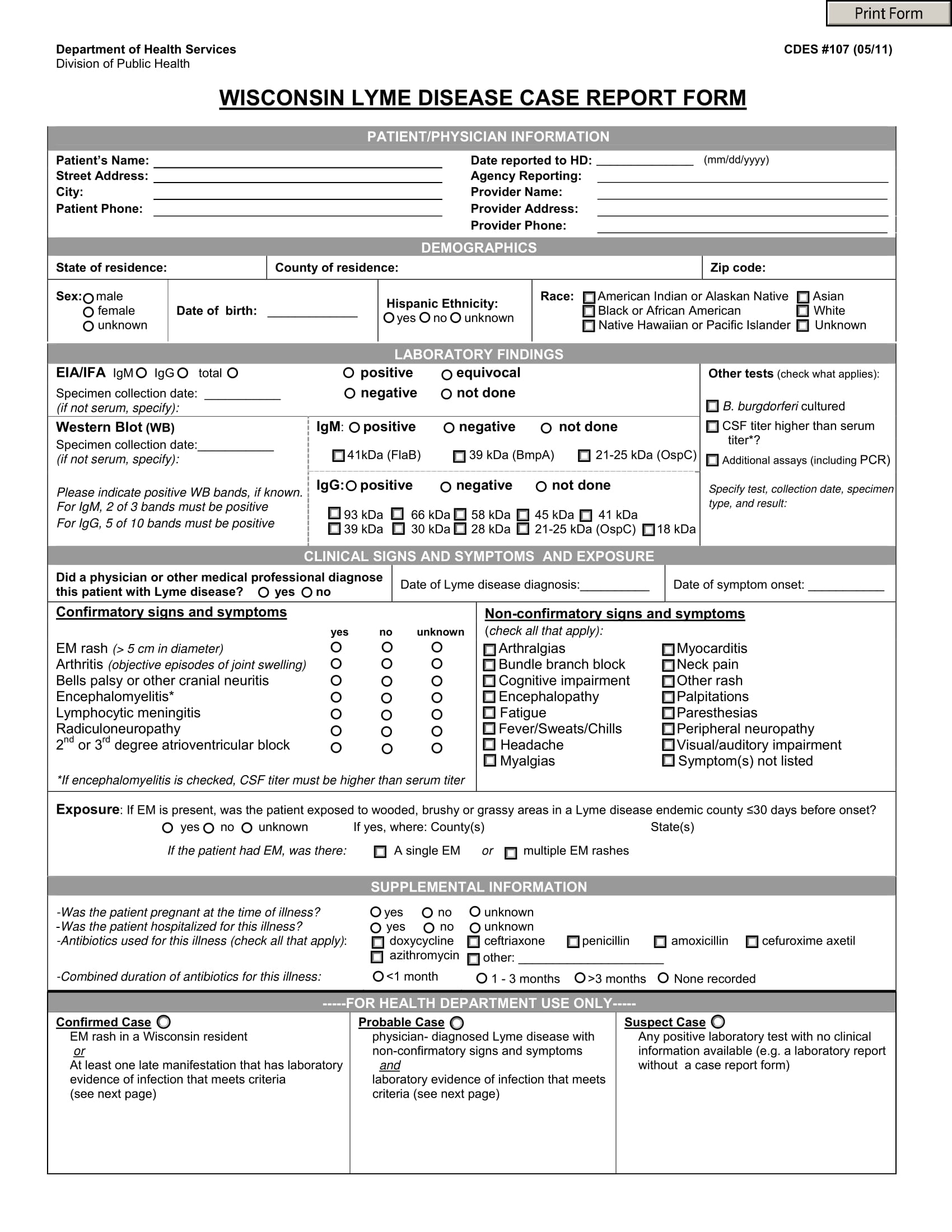
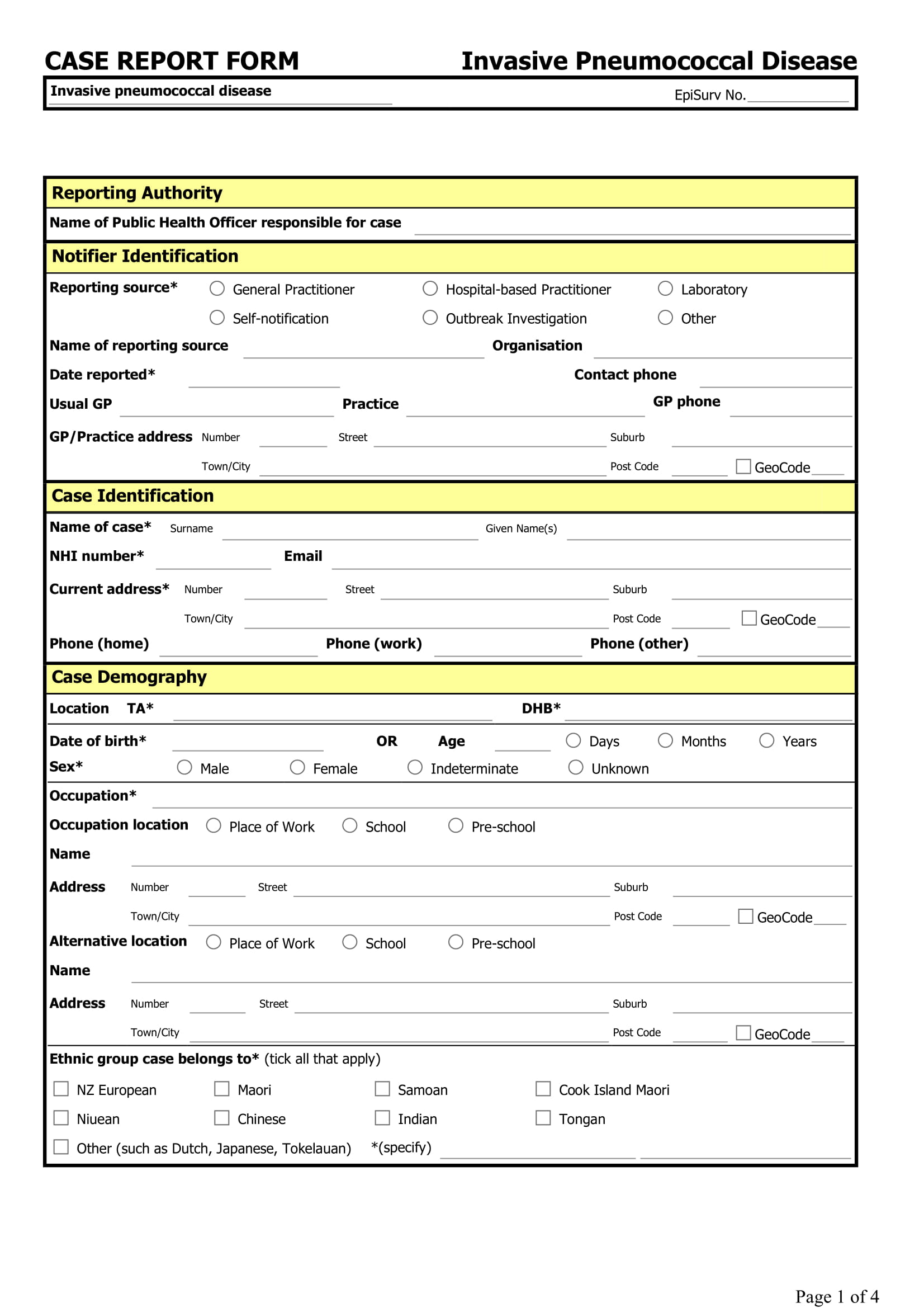
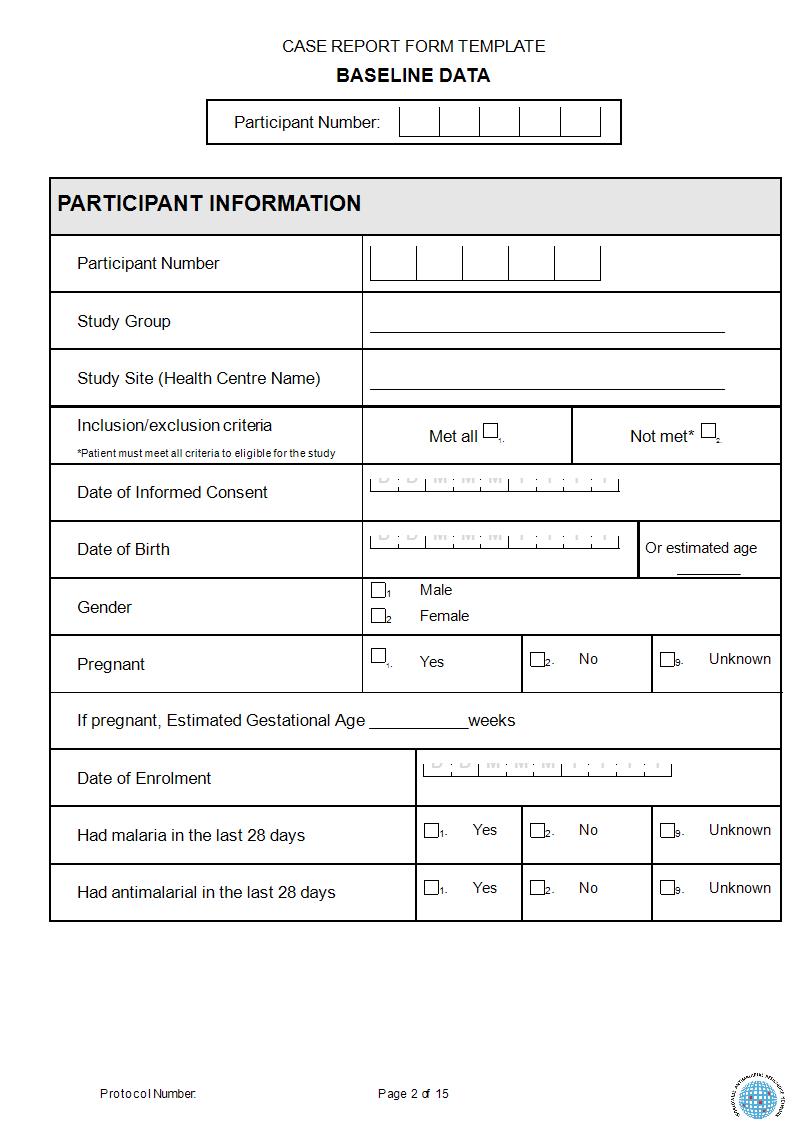
Case Record Form
Case Record Form - Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical. It should be study protocol. Case report form (crf) is a specialized document in clinical research.
It should be study protocol. Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical.
Case report forms (crfs) are critical documents in clinical research that record study data for each. It should be study protocol. Case report form (crf) is a specialized document in clinical research. Learn how to design and develop a reliable and valid case report form (crf) for clinical.
Blank Case Report Form RIAT Support Center
It should be study protocol. Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report forms (crfs) are critical documents in clinical research that record study data for each. Case report form (crf) is a specialized document in clinical research.
Two pages of the case record form as an example. Download Scientific
Case report form (crf) is a specialized document in clinical research. It should be study protocol. Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical.
Free 15+ Case Report Forms In Pdf Doc inside Case Report Form
Case report forms (crfs) are critical documents in clinical research that record study data for each. It should be study protocol. Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report form (crf) is a specialized document in clinical research.
Case Report Form Template
Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are critical documents in clinical research that record study data for each. It should be study protocol.
FREE 15+ Case Report Forms in PDF MS Word
Learn how to design and develop a reliable and valid case report form (crf) for clinical. It should be study protocol. Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are critical documents in clinical research that record study data for each.
FREE 15+ Case Report Forms in PDF MS Word
Case report forms (crfs) are critical documents in clinical research that record study data for each. Case report form (crf) is a specialized document in clinical research. Learn how to design and develop a reliable and valid case report form (crf) for clinical. It should be study protocol.
Case Record Form
Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report forms (crfs) are critical documents in clinical research that record study data for each. It should be study protocol. Case report form (crf) is a specialized document in clinical research.
Case Report Form Template
It should be study protocol. Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report form (crf) is a specialized document in clinical research.
FREE 15+ Case Report Forms in PDF MS Word
Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report forms (crfs) are critical documents in clinical research that record study data for each. It should be study protocol. Case report form (crf) is a specialized document in clinical research.
Sample page of case report form completion guideline Download
It should be study protocol. Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical.
It Should Be Study Protocol.
Case report forms (crfs) are critical documents in clinical research that record study data for each. Learn how to design and develop a reliable and valid case report form (crf) for clinical. Case report form (crf) is a specialized document in clinical research.